medicine
RPM code revisions shelved again
Another proposed revision of billing codes for monitoring patients outside the clinic with wearables and other devices has been shelved, delaying changes that would make it easier for vendors and medical practices to offer more services to patients.
The revisions to remote physiologic monitoring and remote therapeutic monitoring codes were considered at last week’s meeting of the American Medical Association’s CPT Editorial Panel, which includes health care professionals and representatives from medical specialty societies, hospitals, payers, and government. After discussion, the panel decided to postpone a vote on the merits of the revisions while various updates proposed during the meeting could be incorporated. The next editorial panel meeting is in September.
The revisions aimed to expand the existing codes, for example by making it possible to bill for supplying devices when 2 to 15 days of data were transmitted in a month or when 16-30 days were transmitted. Today, providers can only bill with at least 16 days. In addition, the changes would have added codes for remote care management for 11 to 20 minutes a month. Before, providers had to hit 20 minutes to bill.
There's a broad feeling that remote monitoring codes should be updated because they're too restrictive and don't cover a broad class of potentially valuable use cases. For example, it may not be necessary or productive for someone in a weight management program to step on a scale 16 times in a month. But the road to change has been challenging: Revisions to the codes had previously been removed from the editorial panel’s agenda several times, including most recently in February.
At least part of the reason is a desire to make sure revisions don't set the field back. The code changes that had been proposed for the February meeting amounted to a complete overhaul that would have involved deleting and combining existing codes and would have undermined years of data collection by insurers and could have set back progress toward reimbursement.
"There's a lot of people now that are having success billing for RPM," Joseph Kvedar, who leads the AMA's Digital Medicine Payment Advisory Group, told me in February. The advisory group put forth both the original remote monitoring codes and the recent revision proposals. He added: "There is a fear, I think, in the market that if we start monkeying with these codes, that insurance companies will see them as too vague and stop paying for them." (In a report last year, the AMA detailed the spotty coverage for codes among large commercial insurers.)
The revisions considered at the May meeting weren't as aggressive, but it appears that the editorial panel needed more time to consider them. We'll see what happens in September!
Does this impact your business? Do you have thoughts about it? Let me know: mario.aguilar@statnews.com
Medical devices
Abbott dissolvable stent gets the green light
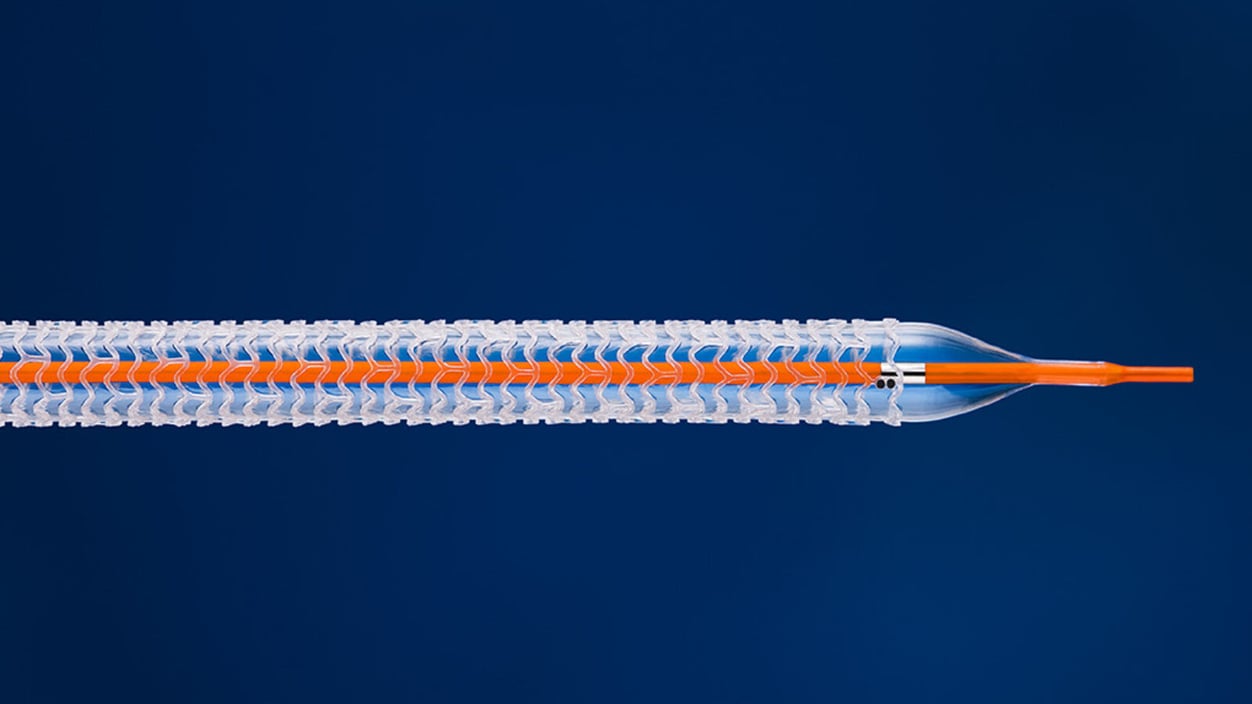
An Abbott device that failed in heart disease patients is getting a new life in patients with severe vascular disease.
The device is a below-knee stent that widens clogged blood vessels, and then vanishes into the vessel’s walls over the course of three years. It also delivers a drug that prevents scar tissue from forming — a common risk factor with traditional metal stents that further narrow the vessel.
Abbott’s first dissolvable stent, for coronary artery disease, received Food and Drug Administration approval in 2016. But the company voluntarily pulled the product from the market just over a year later due to “low commercial uptake.” The device also received mixed results in clinical trials.
Abbott and its associated clinicians are far more confident in the new, thinner stent. Cardiovascular doctors are cautiously optimistic, based on positive clinical trial evidence and a more modern design, that the device will perform better in the leg than it did in the heart. The FDA is, too. The agency approved the product in April, and doctors implanted the device in its first commercial patient in Mount Sinai Hospital last week.
Read more about Abbott's new stent from STAT's Lizzy Lawrence here.
regulation
FDA AI device clearances soar
The Food and Drug Administration this week updated its public list of cleared artificial intelligence and machine learning products with 191 additions, 150 of which received final decisions between August 1 and March 31. The list includes 882 devices so far.
A few observations. First, the agency is clearing AI devices at a blistering pace even as broad standards for evaluating the products is in flux. And, it's interesting that the agency continues to dredge up examples from the past.
Nearly all of the newly added devices were cleared on claims that they were substantially equivalent to previously cleared devices. A large number of the clearances are for uses in radiology.



