Telehealth
Where are patients getting their obesity medications?
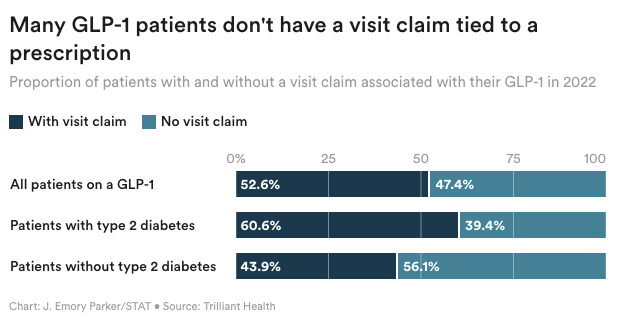
As demand for GLP-1s like Wegovy and Ozempic continues to soar, direct-to-consumer telehealth companies have answered the call for prescription access and weight loss support — and a new analysis suggests that they could account for nearly half of the patients with insurance claims for the prescriptions in 2022. Trilliant Health analyzed commercial and federal claims data for 300 million Americans, and found that 47% of prescription claims for the drug class were missing an associated medical visit claim within three days.
Many of those patients may have paid cash for a telehealth visit with a company like Calibrate or Ro, which don’t accept insurance for visits but help users navigate their benefits to pay for the drugs.
Read more from STAT's Katie Palmer here.
Earnings
Pharma tightening trickles down to Doximity
Health care networking portal Doximity posted better than expected earnings on $108 million in revenue last quarter, an increase of 20% year over year. But it wasn’t all sunny news as the company also lowered its forecast for the year and announced it would lay off about 100 people, or 10% of its workforce.
Doximity’s headwinds around its marketing solutions — which help pharmaceutical and hospital clients advertise their products — are notable. CEO Jeff Tangney told investors that “pharma's shift to digital has slowed” and that “budgetary caution rules the day.” The company said that it saw fewer mid-year “upsells,” attributable to how clients prefer self-service solutions to Doximity’s typical hands-on sales strategy. To remedy this, Doximity is modernizing its advertising technology. “We believe that by following the well-tested self-serve ad platform playbook of other tech companies will unlock [small business] clients that we've never had before,” said Tangney. He added: "While this won't happen overnight, we believe it will also allow us to operate more efficiently as more programs run with the click of a button rather than through our white glove team."
Digital Therapeutics
Pushing forward with digital treatments for gut conditions
In a sign of the growing interest in digitizing behavioral interventions for all kinds of diseases, the latest American Journal of Gastroenterology issue featured a few articles on the potential for digital-based treatments for conditions.
In one, Japanese researchers explored using an app to help people manage nonalcoholic steatohepatitis, a form of fatty-liver disease which can lead to cirrhosis and other complications. The small, single-arm study was conducted over a year. Patients received lifestyle guidance through the app on top of usual outpatient care, and 68% saw improvement in their disease scores. The intervention would need more thorough study for conclusive results, but the research illustrates just how many interventions might be supplemented with apps.
Another paper, from authors at Michigan Medicine, gives a useful overview of existing apps for the treatment of irritable bowel syndrome, including products from Mahana Therapeutics, metaMe Health, Bold Health, and Mindset Health. Though the paper highlights the peer-reviewed evidence for each app, it stops short of passing judgment. Author William Chey, a professor and chief of the division of gastroenterology and hepatology at Michigan, told STAT by email that “digital therapeutics should be held to the same standard as face-to-face interventions.” Chey describes himself as an “early adopter of integrated, multispecialty care for patients with digestive disorders,” and said his department already recommends digital therapeutics to patients “who prefer them for whatever reason or as an adjunct to seeing our behavioral therapists.”